Location
The Robert J. Bernard Field Station (BFS) is located at the foot of the San Grabriel Mountains on the eastern edge of Los Angeles County in Claremont CA (Figure 1). The BFS harbors multiple terrestrial habitat types including: native California sage scrub (25 ha), a small non-native grassland (3 ha), two burned areas resulting from fires in 2013 (5.5 ha) and 2017 (2 ha) (see Vegetation section below for more details). In addition, the BFS harbors an artificial lake (pHake Lake) that has a unique assemblage of vascular plants in and adjacent to the lake. The natural habitats at the BFS are surrounded by a mixture of modified suburban habitat types1-4. Fragmentation of natural areas into “islands” of habitat surrounded by an urban/suburban “sea” is common in low elevation areas of southern California1-8. Because the BFS harbors or is adjacent to many of the common habitat types across southern California (California sage scrub, non-native grasslands, and a diversity of urban/suburban habitats), and experiences many stressors common in urbanized southern California (isolation via fragmentation, urban heat effects, and increased frequency of fires) it offers an ideal microcosm to explore how habitat modifications and other disturbances influence biodiversity and key ecosystem services as has been the focus of many senior theses over the past decade1-4, 9-15.
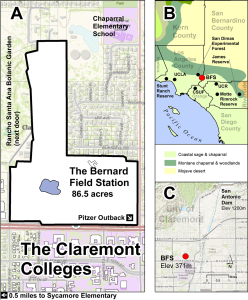
Figure 1. Location of the Bernard Field Station in Claremont CA north of the Claremont Colleges (A), its location in the region (B), and its location relative to other natural areas in the Claremont foothills (C).
Climate
The BFS experiences a Mediterranean Climate. Mediterranean Climates are defined by having two seasons: (1) a hot-dry season, and (2) a cool-moist season16. Examining the tables below, you can see a majority of the rainfall occurs during the cooler months (November to April), while there is little rainfall during the warmer months (May to October). Other than California, Mediterranean Climate Ecosystems are found in southern Chile, the tip of South Africa, southwestern Australia, and around the Mediterranean Basin, all biodiversity hotspots17.
Table 1. Average monthly temperatures (°C) over the last 5 years.
| Year | ||||||
| Month | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 |
| January | 11.6 | 11.7 | 12.0 | 12.5 | 9.8 | 11.1 |
| February | 8.3 | 13.1 | 12.3 | 13.1 | 9.6 | 10.8 |
| March | 12.8 | 11.6 | 11.7 | 14.8 | 10.2 | 11.8 |
| April | 15.9 | 15.0 | 15.7 | 16.1 | 14.8 | 13.9 |
| May | 14.3 | 19.1 | 16.8 | 16.9 | 15.0 | 15.1 |
| June | 19.2 | 20.0 | 21.9 | 22.8 | 16.7 | 20.8 |
| July | 23.4 | 23.3 | 24.7 | 23.5 | 25.2 | 25.5 |
| August | 24.2 | 25.7 | 24.2 | 25.9 | 24.6 | 24.8 |
| September | 22.4 | 24.9 | 22.5 | 26.4 | 20.1 | 29.9 |
| October | 19.0 | 21.3 | 17.4 | 18.8 | 19.5 | 20.5 |
| November | 15.2 | 14.9 | 17.0 | 12.4 | 15.2 | 13.5 |
| December | 11.0 | 12.4 | 10.1 | 10.7 | 12.9 | |
Table 2. Monthly rainfall totals (mm) over the last 5 years.
| Year | ||||||
| Month | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 |
| January | 173 | 5 | 5 | 3 | 222 | 60 |
| February | 240 | 7 | 1 | 8 | 150 | 334 |
| March | 103 | 22 | 4 | 43 | 249 | 98 |
| April | 7 | 41 | 1 | 19 | 21 | 32 |
| May | 55 | 3 | 0 | 6 | 38 | 17 |
| June | 2 | 2 | 0 | 5 | 13 | 0 |
| July | 0 | 0 | 6 | 0 | 0 | 0 |
| August | 0 | 0 | 0 | 0 | 103 | 0 |
| September | 2 | 0 | 0 | 35 | 11 | 0 |
| October | 0 | 0 | 25 | 21 | 6 | 6 |
| November | 83 | 9 | 0 | 108 | 15 | 3 |
| December | 22 | 2 | 250 | 105 | 39 | |
For humans, Mediterranean Climates represent attractive places to live18,19. In southern California and northern Mexico, which have double the population density of any other Mediterranean region20, the resulting development and habitat modifications mean that many of the native ecosystems are some of the most threatened in North America21,22.
For vegetation, Mediterranean Climates represent significant challenges. The biggest challenge is that the supply of water and the need for water are out of phase. Consequently, the growing season in southern California is during the cool-wet season when water resources are available but when cool temperatures slow photosynthesis16. During the hot-dry season, growth is often limited by the lack of available water resources. To survive, plants have evolved different strategies. Three of the most common strategies are: (1) sclerophyllous evergreen plants, which have deep root systems and thick waxy leaves that position themselves parallel to sunlight (e.g., Rhus integrifolia and Heteromeles arbutifolia), (2) drought-deciduous plants that go dormant during the hot-dry summer (e.g., Artemisia californica and Ribes aureum) and (3) annuals that grow when conditions are good, then set seed allowing them to “over-winter” during the hot-dry season.
In addition to using the tables above, you can access weather data using:
- The RAWS Climate Archive
- The weather station data on the C-flux monitor at the BFS
Geology
The BFS lies on the alluvial fan of San Antonio Creek. Consequently, the sediment is composed primarily of debris of various sizes that washed out of the mountains during heavy rains and floods prior to the construction of the San Antonio Dam. This explains the presence of smooth pebbles, cobbles, and rounded boulders (Claremont Potatoes) at the BFS and throughout Claremont. Because soil (sediments less than 2 mm in diameter) at the BFS is primarily derived from eroded sediments washed out of the San Gabriel Mountains during high flow events and are weakly developed because they are young, BFS soils are composed primarily of sand (80% or higher) with small concentrations of silt (10 to 15%) and clay (approximately 5%)9,11,15,23. Using USDA NRCS Soil Taxonomy, soils at the BFS are best classified as mixed, sandy-skeletal Humic Haploxerepts (Inceptisols)9-24.
While there are no differences in sand, slit, and clay concentrations across habitats at the BFS, there are differences in other soil properties highlighting that different plant assemblages can influence many soil properties9,11,15,23. Notably, pH seems to be elevated in the non-native grassland and in areas dominated by non-native mustards, while sage scrub soils are more acidic9,11,15,23. In addition, C and N concentrations are higher in sage scrub soils than in the non-native grassland highlighting that modifications in plant community structure can have significant influence on soil properties, and more importantly, soil C storage capacity9,11,23.
However, it is important to note that small areas of the BFS have been impacted by past land use activities (e.g., grazing, vehicle travel, and light construction) including the installation of an artificial lake (pHake Lake). Soil physical and chemical properties may be quite different in these areas from the overall character of the BFS soil landscape9.
Vegetation
The BFS vegetation can be classified in various ways as all classification systems are just human constructs developed to try to explain complex patterns in nature. Here, I (WMM – current BFS director) describe how I classify habitats across the BFS.
The primary habitat at the BFS is California sage scrub25. Others may refer to this habitat as coastal sage scrub16, but I prefer to use Rundell’s25 (a Pomona College Alum) classification system as the use of “California” provides the location of where this ecosystem type can be found. In addition, Rundel’s classification, breaks California sage scrub into four different associations: coastal sage scrub (located ~ 10 km from a coast), interior sage scrub (BFS and other inland sites), maritime succulent sage scrub (arid coastal regions in Baja California), and island sage scrub (sage scrub assemblages composed of plants that became established on the California Channel Islands). Because Rundel uses the term coastal sage scrub to describe a component of this ecosystem not found at the BFS, I feel compelled to use California sage scrub to reduce ambiguity. However, if you use Ecosystems of California as your source16, then coastal sage scrub is the correct term for this habitat type. I recommend whichever term you use in your research, that you spend extra time describing the assemblage of plants that compose the BFS sage scrub, or your study area, so the reader can better interpret the system you are working in as, you will find that plant assemblages differ significantly across sites in southern California1,11.
The BFS harbors a relatively intact piece (25 ha) of California sage scrub, which is characterized by being composed of native drought-deciduous shrubs (Artemisia californica and Ribes aureum) and other small-statured (~ 1 m in height) drought-tolerant evergreen shrubs (e.g., Eriodictyon trichocalyx, Eriogonum fasciculatum, Salvia apiana, Ericameria pinifolia)16,25. Because the BFS is located near the San Gabriel Mountains, increased orographic precipitation also supports larger chapparal-type shrubs (e.g. Malosma laurina, Rhus integrifolia, Heteromeles arbutifolia) that are more common in sage scrub areas near the coast and at higher elevations where the chaparral ecosystem is more common25. In addition, the sage scrub at the BFS supports a wide variety of annual and perennial forbs: e.g., Penstemon spectabilis, Eriastrum sapphirinum, Cirsium occidentale, Camissoniopsis bistorta, and Cryptantha spp.. See the BFS plant list for a complete list of all the plants known to the BFS.
The BFS also harbors a small (~ 3.5 ha) non-native grassland in the northeastern field. While there were once native grasslands in southern California dominated by perennial native grasses and forbs, occasionally referred to as the California Prairie, this ecosystem is extinct16. Now most grasslands in California, including the one at the BFS, are dominated by non-native annual grasses, mostly Bromus spp. (Bromus diandrus and Bromus rubens), but also Avena spp. and Schismus barbatus11,23. Non-native forbs, Erodium spp. and mustards, mostly Hirschfeldia incana in our area15, also can be found in this habitat. While some native plants persist in the non-native grassland, including Amsinckia intermedia, Eriogonum gracile, Datura wrightii, and Croton setiger, plant diversity is typically lower in this habitat than in sage scrub. The non-native grassland at the BFS has persisted for more than 40 years following the removal of a citrus grove, which has been shown to create soil legacy effects that may favor non-native grasses and limit CSS shrub recolonization (Hilbig 2015).
In addition to these two habitats, the BFS harbors a 5.5 ha area that has been described as a transitional habitat, an area that once had been cleared but has sage scrub shrubs recolonizing this area1,2. Consequently, it represents an ecotone between the intact sage scrub area and the more disturbed non-native grassland. In September 2013, a human-ignited fire burned ~ 7 ha encompassing the entire transitional area and small parts of the adjacent sage scrub10,13,27. Because of the fire, this area is often referred to as the burned area. However, another fire in 2017 burned ~ 2 ha of sage scrub and lake vegetation to the east and north of the 2013 fire area28. Now, many people refer to these areas as the 2013 fire area and the the 2017 fire area. Studies in these areas have tracked how vegetation and animals are recovering following the fires2,10,13,14,27-29.
These represent the primary terrestrial habitat types at the BFS. However, others have described other smaller habitats to highlight differences that occur at smaller scales. For example, there is a small area in the center of the BFS that supports a grove of Coast Live Oak (Quercus agrifolia). Above the oak grove, Wakefield et al.15 described a small area as a non-native forbland or mustard-land, as disturbance in this area created conditions for it to be colonized by the short-pod mustard (Hirschfeldia incana). Similarly, areas directly adjacent to the lake have different plant assemblages, though it is unclear if differences are due to different abiotic aspects (more water and differing soil structure) facilitating the recruitment of different plants species, if plants were planted following construction of the pHake lake, or both13,30.
In addition, many have highlighted that the sage scrub is different in the neck than in more southern areas of the BFS. Presence of Coastal Live Oaks and Sycamores (Platanus racemosa), both large statured trees, along with Water Wally (Baccharis emoryi), suggest that a permanent ground water source may be closer to the surface here than in other parts of the BFS which may help explain why assemblages may differ.
References
1Staubus* W. J., E. S. Boyd, T. A. Adams, D. M. Spear, M. M. Dipman and W. M. Meyer III. 2015. Ant communities in native sage scrub, non-native grassland, and suburban habitats in Los Angeles County, USA: conservation implications. Journal of Insect Conservation 19: 669-680.
2Spear, D. M., E. S. Boyd, T. A. Adams, M. M. Dipman, J. W. Staubus and W. M. Meyer III. 2017. The effects of development, type conversion, and fire on low-elevation California spider assemblages. Invertebrate Biology 136: 134-145.
3Staubus, W. J., S. Bird, S. Meadors, and W. M. Meyer III. 2019. Distributions of invasive arthropods across heterogeneous urban landscapes in southern California: aridity as a key component of ecological resistance. Insects 10: 29.
4Vourlitis, G.L., E. L. van der Veen, S. Cangahuala, G. Jaeger, C. Jensen, C. Fissore, E. M. Wood, J. K. Abraham, K. S. Whittemore, E. Slaven, D. VanOverbeke, J. Blauth, E. Braker, N. Karnovsky, and W. M. Meyer III. 2022. Examining decomposition and nitrogen mineralization in five common urban habitat types across southern California to inform sustainable landscaping. Urban Science 6: 61.
5Alberts, A. C., A. D. Richman, D. Tran, R. Sauvajot, C. McCalvin, and D.T. Bolger. 1993. Effects of habitat fragmentation on populations of native and exotic plants in southern California coastal scrub. Pages 103–110 in J. E. Keely (ed) Interface between Ecology and Land Development in California. Southern California Academy of Science, Los Angeles, CA.
6Bolger, D. T., A. V. Suarez, K. R. Crooks, S. A. Morrison, and T. J. Case. 2000. Arthropods in urban habitat fragments in Southern California: area, age, and edge effects. Ecological Applications 10:1230-1248.
7Soule´, M. E., D. T. Bolger, A. C. Alberts, R. Sauvajot, J. Wright, M. Sorice, and S. Hill. 1988. Reconstructed dynamics of rapid extinction of chaparral requiring birds in urban habitat islands. Conservation Biology 2:75-92.
8Suarez, A. V., D. T. Bolger, and T. J. Case. 1998. The effects of fragmentation and invasion on the native ant community in coastal southern California. Ecology 79:2041-2056.
9Wheeler, M. M., M. M. Dipman, T. A. Adams, A. V. Runia, C. R. Robins, and W. M. Meyer III. 2016. Carbon and nitrogen storage in California sage scrub and non-native grasslands. Journal of Arid Ecosystems 129: 119-125.
10Adams, T. A., J. W. Staubus, and W. M. Meyer III. 2018. Fire impacts on ant assemblages in California sage scrub. Southwestern Entomologist 43: 323-334.
11Caspi, T., L. A. Hartz, A. E. Soto Villa, J. A. Loesberg, C. R. Robins, and W. M. Meyer III. 2019. Impacts of invasive annuals on soil carbon and nitrogen storage in southern California depend on the identity of the invader. Ecology and Evolution 9: 4980-4993.
12Dipman, M. M., and W. M. Meyer III. 2019. Type conversion from native California sage scrub to non-native grassland accelerates decomposition processes. Applied Soil Ecology 144: 68-71.
13Dartnell, S, N. Hamlett, and W. M. Meyer III. 2022. Monitoring butterfly assemblages in southern California to assess the impact of habitat and climate modifications. Journal of Insect Conservation 26: 149-262.
14Jones, I., and W. M. Meyer III. 2023. Acmispon glaber shrub canopies facilitate Bromus madritensis establishment after fire in California sage scrub. Bulletin, Southern California Academy of Sciences 122: 158-163.
15Wakefield, Z. R., A. R. O. Cavalcanti, L. Driessen, A. Jaramillo, E. J. Crane III, G. Richetta and W. M. Meyer III. 2023. Effects of mustard invasions on soil microbial abundances and fungal assemblages in southern California. Diversity 15: 50.
16Zavaleta, E., and H. Mooney, eds. 2016. Ecosystems of California. University of California Press, Oakland, CA.
17Myers N, R. A. Mittermeier, C. G. Mittermeier, G. A. B. de Fonseca, and J. Kent. 2000. Biodiversity hotspots for conservation priorities. Nature 403:853–858.
18Sala O. S., F. S. Chapin III, J. J. Armesto, E. Berlow, J. Bloomfield, R. Dirzo, E. Huber-Sannwald, L. F. Huenneke, R. B. Jackson, A. Kinzig, R. Leemans, D. M. Lodge, H. A. Mooney, M. Oesterheld, N. L. Poff, M. T. Sykes, B. H. Walker, M. Walker, and D. H. Wall 2000. Global biodiversity scenarios for the year 2100. Science 287:1770-1774.
19Cox R. L., and E. C. Underwood 2011. The importance of conserving biodiversity outside of protected areas in Mediterranean ecosystems. PLoS One 6:e14508
20Underwood, E. C., J. H. Viers, K. R. Klausmeyer, R. L. Cox, and M. R. Shaw. 2009. Threats and biodiversity in the Mediterranean biome. Diversity and Distributions 15:188-197
21Noss, R. F., E. T. LaRoe III, and M. J. Scott. 1995. Endangered Ecosystems of the United States: A Preliminary Assessment of Loss and Degradation. Biological Report 28. National Biological Service, U.S. Department of Interior, Washington, DC. USA.
22Riordan, E.C., P. W. Rundel. 2014. Land use compounds habitat losses under projected climate change in a threatened California ecosystem. PLoS One 9:e86487
23Caspi* T., L. Estrada, A. V. Dowling, E. Su, M. Leshchinskiy, A. R. O. Cavalcanti, E. J. Crane, C. R. Robbins, and W. M. Meyer III. 2018. Carbon and nitrogen in the topsoils of Inceptisols and Mollisols under native sage scrub and non-native grasslands in southern California. Geoderma Regional 14: e00172.
24Soil Survey Staff, 2014. Keys to Soil Taxonomy, 12th edition. United States Department of Agriculture Natural Resources Conservation Service.
25Rundel, P. W. 2007. Sage scrub. In: Barbour MG, Keeler Wolf T, Schoenherr AA (eds) Terrestrial Vegetation of California. University of California Press, Berkeley, pp 208-228
26Hilbig, B. 2015. Ecological assembly rules and soil legacy effects in the restoration of an invaded plant community. Dissertation, University of California Riverside.
27 Thomson, D. M., W. M. Meyer III, and I. F. Whitcomb. 2021. Non-native plant removal and high rainfall years promote post-fire recovery of Artemisia californica in southern California sage scrub. PLoS ONE 16: e0254398.
28Meyer, W.M., III, C. Halligan, L. Thomey, K. Madunich-Arévalo, C. Parry, R. Scaff, R. Macy, I. Jones, E. Halligan, A. Jaramillo, A. N. T. Phan, S. Thierry, E. J. Crane III, and A. R. O. Cavalcanti. 2022. Herbivore influence on post-fire California sage scrub plant and soil microbial assemblages. Diversity 14: 1110.
29Thomson, D.M. 2024. Using demographic modeling to develop post‐fire restoration strategies for a native shrub in a sage scrub community. Restoration Ecology p.e14274.
30Dartnell, S., A. R. O. Cavalcanti, A. Misaki Bluebell, N. V. Hamlett, E. J. Crane III, W. M. Meyer III. 2022. Flower-visiting insect assemblages on fall-blooming native California sage scrub shrubs. Diversity 14: 958.
